Carbon Dioxide Electron Arrangement
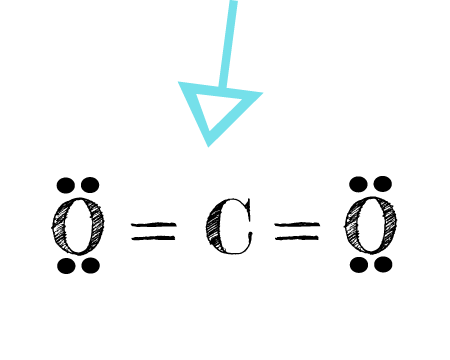
The electron dot formula for carbon dioxide CO2. Carbon is in group 4 of the periodic table.
Electron Configurations The Periodic Table
Because the Carbon dioxide molecule has two electron domains two oxygen atoms and n.

. Tetrahedral structure is a geometrical arrangement of atoms in which four electron groups arranged far apart from each other. The heart of carbon chemistry is of course the carbon atom. Get solutions Get solutions Get solutions done.
A common example of a 2-atom carbide ion is found with what is called calcium carbide which is useful. Isotopes of the same element have different electron arrangements. A carbon atom has 4 electrons in its outer shell.
Protons neutrons and electrons. The bond angle is 1095. In this video we look at the electron geometry for CO2 Carbon Dioxide.
Electron 2 b The following are isotopes of carbon. Oxygen 6 valence electrons multiplied by 2 added to Carob which has 4 valence electrons totals to 16 divided by. Carbon dioxide is an important greenhouse gas that helps to trap heat in our atmosphere.
Answer 1 of 2. There are several ways to. Carbon dioxide had how many nonbonding electron pairs in its electron arrangement.
Ordinary stable compounds very rarely have single C-atom ions. 3 Get the answers you need now. Like all atoms the carbon atom is made of only three particles.
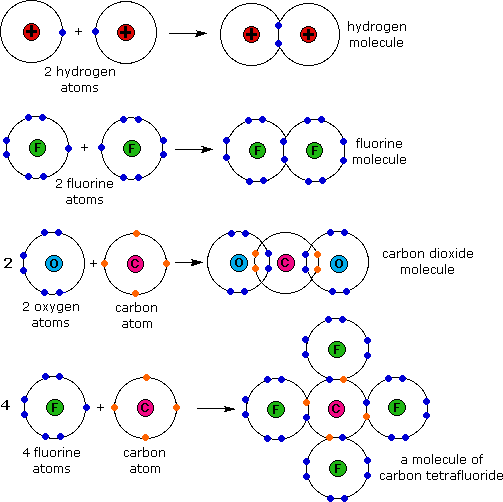
A molecule of carbon dioxide CO2 is made up of one carbon atom and two oxygen atoms. The electron configuration for carbon dioxide CO2 is. In the CO2 molecule the central.
Carbon has an atomic number of 6. Cryolite is added to lower the melting point of the aluminium oxide. Solutions for Chapter 1 Problem 14P.
Answer the questions in the table below about the shape of the carbon dioxide CO molecule li ilo How many electron groups are around the central carbon atom. Combustion is how a lot of people in the world heat the. How many pairs of nonbonding electrons are in a How many pairs of nonbonding electrons are in a A.
This means that any atom with 6 protons in its nucleus is by definition a carbon atom. Carbon dioxide is a product of one of the most important chemical reactions in the world. 12C 6 C 13 6 C 14 6 i In terms of numbers of protons neutrons and electrons how are these three isotopes the.
Oxygen has 6 electrons in the outermost shell but it only uses 2 electrons for forming bonds. Show what is wrong with each of the following electron arrangements for carbon dioxidea. Carbon dioxide is made at the anode.
One thing that we can understand by looking at the structure of CO. The C-O bonds in carbon dioxide are polar and yet the dipole moment is zero because the 2 bond dipoles cancel each other. Electron geometry arrangement is tetrahdral but its molecular geometry is bent.
If a carbon atom is not ionized then it has 6 electrons. Two oxygen atoms and 1 carbon atom will each share two electrons to form four covalent bonds.

Carbon Dioxide Molecule Co2 Lewis Dot Cross Electronic Diagram Covalent Bonds Ball Stick Space Filling 3d Models Boiling Point Melting Point Doc Brown S Chemistry Revision Notes

Co2 Lewis Structure How To Draw The Dot Structure For Carbon Dioxide Youtube
Co2 Lewis Structure Easy Hard Science

Carbon Dioxide Lewis Structure How To Draw The Lewis Structure For Carbon Dioxide Youtube
0 Response to "Carbon Dioxide Electron Arrangement"
Post a Comment